BIOLOGY
Theory Paper Time : 3 Hours Max. Marks: 70
Practical Time : 3 Hours Max. Marks 30
Total Marks : 100
THEORY
STRUCTURE OF QUESTION PAPER
1. There will be one theory paper comprising of 30 questions. All questions will be compulsory.
2. Marks for each question are indicated against it.
3. Q Nos. 1-10 are very short answer type questions carrying 1 mark each. Answer to each question will be in one line or few words only.
4. Q.Nos. 11-18 are short answer type questions carrying 2 marks each. Answer to each question will be in 20 to 30 words.
5. Q. No. 19-26 are short answer type questions carrying 3 marks each. Answer to each question will be in 40 to 50 words.
6. Q. No. 27to 30 are long answer type questions carrying 5 marks each. Answer to each qustion will be in 80 to 100 words.
7. In Q. No. 27 to 30, there will be 100% internal choice.
8. Distribution of marks to cover different dimensions of question paper will be as under: Learning outcomes Marks Percentage of Marks
i) Knowledge 20 29%
ii) Understanding 30 42%
iii) Application 20 29%
9. There will be no objective type questions such as ‘yes/no’, tick/cross’, fill in the blanks multiple choice, true/false etc.
10. The question paper should be strictly from the prescribed syllabus based inabove mentioned guidelines.
The unit wise distribution of marks will be as follows:
Unit - I Sexual Reproduction 12 Marks
Unit - II Genetics and Evolution 20 Marks
Unit - III Biology and Human Welfare 12 Marks
Unit -IV Biotechnology and Its Applications 12 Marks
Unit - V Ecology and Environment 14 Marks
Unit I : Sexual Reproduction
Pollination and fertilization in flowering plants. Development of seeds and fruits. Human reproduction : reproductive system in male and female, menstrual cycle, production of gametes, fertilization,implantation, embryo development, pregnancy and parturition.
Reproductive health:birth control, contraception and sexually transmitted diseases.
Unit II : Genetics and Evolution
Mendelian inheritance. Chromosome theory of inheritance, deviations from Mendelian ratio (gene interactionincomplete
dominance, co-dominance, complementary genes, multiple alleles). Sex determination in human beings : XX, XY.
Linkage and crossing over. Inheritance pattern of haemophilia and blood groups in human beings.
DNA: replication, transcription, translation. Gene expression and regulation.
Genome and Human Genome Project. DNA fingerprinting. Evolution : Theories and evidences.
Unit III Biology and Human Welfare
Animal husbandry. Basic concepts of immunology, vaccines. Pathogens and parasites. Plant breeding, tissue culture, food production. Microbes in houshold food processing, industrial production, sewage treatment and energy generation.
Cancer and AIDS. Adolescence, drugs and alcohol abuse.
Unit IV Biotechnology and Its Applications
Recombinant DNA technology. Applications in Health, Agriculture and Industry. Genetically modified (GM) organizms; biosafety issues. Insulin and Bt cotton.
Unit V Ecology and Environment
Ecosystems components, types and energy flow. Species, population and community. Ecological adaptations.
Centers of diversity and conservation of biodiversity, National Parks and Wild Life Sanctuaries. Environmental issues.
CHEMISTRY SYLLABUS (10+2)
Theory Paper Time : 3 Hours Max. Marks: 70
Practical Paper Time : 3 Hours Max. Marks 30
Total Marks : 100
THEORY
STRUCTURE OF QUESTION PAPER
1. There will be one Theory Paper comprising of 30 questions. All questions will be compulsory.
2. Q Nos. 1-10 will be of 1 marks, Q.Nos. 11-18 will be of 2 marks each Q. No. 19-26 will be of 3-marks each, Q-Nos. 27 to 30 will be of 5 marks each.
3. In questions No. 27-30 there will be 100% choice.
4. Distribution of approximate percentage over different dimension in the question paper will be as follows:
i) Knowledge 30%
ii) Understanding 40%
iii) Application 30%
5. Numerical problem will be set in any type of question, however the total weightage to numerical problem will be around 20%.
6. There will be no question of the type. “Write short note on”, and objective type questions such as “Yes/No”, tick ( P) cross (x) fill in blanks, multiple choice,” true/false etc.
7. Use of log tables/unprogrammable calculator is allowed.
8. A candidate will be provided with one answer book of 32 pages only. No extra contineous sheet will be provided.
Unit wise distribution of marks is as follows:
CLASS XII (THEORY)
---------------------------------------------------------------------------------------------------------------------------------------------------------
One Paper Time : 3 Hours 70 marks
---------------------------------------------------------------------------------------------------------------------------------------------------------
Unit No. Title Marks
---------------------------------------------------------------------------------------------------------------------------------------------------------
Unit I Solid State 4
Unit II Solutions 5
Unit III Electrochemistry 5
Unit IV Chemical kinetics 5
Unit V Surface Chemistry 4
Unit VI General principles and processes of Isolation of Elements 3
Unit VII p-Block Elements 8
Unit VIII d-and f-Block Elements 5
Unit IX Coordination Compounds 3
Unit X Haloalkanes and Haloarenes 4
Unit XI Alcohols, Phenols and Ethers 4
Unit XII Aldehydes, Ketones and Carboxylic acids 6
Unit XIII Organic Compounds containing Nitrogen 4
Unit XIV Biomolecules 4
Unit XV Polymers 3
Unit XVI Chemistry in Everyday life 3
Total : 70
---------------------------------------------------------------------------------------------------------------------------------------------------------
Unit I: Solid State (Periods 12)
Classification of solids based on different binding forces: molecular, ionic, covalent and metallic solids, amorphous and crystalline solids (elementary idea), unit cell in two dimensional and three dimensional lattices, calculation of density of unit cell, packing in solids, voids, number of atoms per unit cell in a cubic unit cell, points defects, electrical and
magnetic properties.
Unit II: Solutions (Periods 12)
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in liquids, solid solutions, colligative properties - relative lowering of vapour pressure, elevation of B.P., depression of freezing point, osmotic pressure, determination of molecular masses using colligative properties, abnormal molecular mass.
Unit III: Electrochemistry (Periods 14)
Redox reactions, conductance in electrolytic solutions, specific and molar conductivity variations of conductivity with concentration, Kohlrausch’s Law, electrolysis and laws of electrolysis (elementary idea), dry cell electrolytie cells and Galvanic cells; lead accumulator, EMF of a cell, standard electrode potential, Nernst equation and its application to chemical cells, fuel cells; corrosion.
Unit IV: Chemical Kinetic (Periods 12)
Rate of a reacation (average and instantaneous), factors affecting rates of reaction; concentration, temperature, catalyst; order and molecularity of a reaction; rate law and specific rate constant, intergrated rate equations and half life (only for zero and first order reactions); concept of collision theory (elementary idea, no mathematical treatment)
Unit V: Surface Chemistry (Periods 8 )
Adsorption - physisorption and chemisorption; factors affecting adsorption of gases on solids; catalysis; homogenous and heterogeneous, activity and selectivity; enzyme catalysis; colloidal state: distinction between true solutions, colloids and suspensions; lyophilic, lyophobic, multimolecular and macromolecular colloids; properties of colloids; Tyndall effect, Brounian movenment, electrophoresia, coagulation; emulsion types of emulsions.
Unit VI: General Principles and Processes of Isolation of Elements (Periods 8 )
Principles and methods of extraction - concentration, oxidation, reduction electrolytic method and refining; occurrence and principles of extraction of aluminium, copper, zinc and Iron.
Unit VII: p-Block Elements (Periods 14)
Group 15 elements: General introduction, electronic configuration, occurrence, oxidation states, trends in physical and chemical properties; nitrogen - preparation, properties and uses; compounds of nitrogen: preparation and properties of ammonia and nitric acid, oxides of nitrogen (structure only); Phosphorous-allotropic forms; compounds of phosphorous:
preparation and properties of phosphine, halides (PCI3, PCI5) and oxoacids (elementary idea only).
Group 16 elements: General introduction, electronic configuration, oxidation states, occurrence, trends in physical and chemical properties; dioxygen; preparation, properties and uses; simple oxides; Ozone, Sulphur - allotropic forms; compounds of sulphur; preparation, properties and uses of sulphur dioxide sulphuric acid: industral process of manofacture, properties and uses, oxoacids of sulphur (structures only).
Group 17 elements : General introduction, electronic configuration, oxidation states, occurrence, trends in physical and chemical properties; compounds of halogens; preparation, properties and uses of chlorine and hydrochloric acid, interhalogen compounds, oxoacids of halogens (structures only).
Group 18 elements: (General introduction, electronic configuration. Occurrence, trends in physical and chemical properties, uses.
Unit VIII: d and f Block Elements (Periods 14)
General introduction, electronic configuration, occurrence and characteristics of transition metals, general trends in properties of the first row transition metals metallic character, ionization enthalpy, oxidation states, ionic radii, colour catalytic property, magnetic propertics, interstitial compounds, alloy formation. Preparation and propertics of K2Cr2O7 and KMnO4.
Lanthanoids-electronic configuration, oxidation states, chemical reactivity and lanthanoid contraction.
Actionoids - Electronic configuration, oxidation states.
Unit IX: Coordination Compounds (Periods 12)
Coordination compounds - introduction, ligands, coordination number, colour, magnetic properties and shapes, IUPAC nomenclature of mononuclear coordination compounds bonding; isomerism, importance of coordination compounds (in qualitative analysis, extraction of metals and biological systems).
Unit X: Haloalkanesa and Haloarenes. (Periods 12)
Haloalkanes:
Nomenclature, natuer of C-X bond, physical and chemical properties, mechanism of substitution reactions.
Haloarenes:
Nature of C-X bond, substitution reactions (directive influence of halogen for monosubstituted compunds only)
Uses and environmental effects of - dichloro methane, trichloromethane, tetrachloromethane, iodoform, freons, DDT.
Unit XI: Alcohols, Phenols and Ethers (Periods 12)
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary alcohols only); identification of primary, secondary and teritary alcohols; mechanism of dehydration, uses, some important compounds - methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of phenol, electrophillic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit XII: Aldehydes, Ketones and Carboxylic Acids (Periods 12)
Aldehydes and Ketones: Numenclature, nature of carbonyl group, methods of preparation, physical and chemical properties, and mechanism of nuclcophilic addition, reactivity of alpha hydrogen in aldehydes; uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical properties; uses.
Unit XIII: Organic compounds containing Nitrogen (Periods 10)
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical properties, uses, identification of primary, secondary and teritary amines. Cyanides and Isocyanides - will be mentioned at relevant places in context.
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Unit XIV: Biomolecules (Periods 8 )
Carbohydrates - Classification (aldoses and keloses), monosaccahrides (glucose and fructose), oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose, glycogen); importance.
Proteins - Elementary idea of a-amino acids, peptide bond, polypeptides proteins, primary structure, secondary structure, teritary structure and quaternary structure (qualitative idea only), denaturation of proteins; enzymes.
Vitamins - Classification and functions.
Nucleic Acids: DNA & RNA.
Unit XV: Polymers (Periods 8 )
Classification - natural and synthetic, methods of polymerization (addition and condensation), copolymerization. Some importance polymers; natural and synthetic like polythene, nylon, polyesters, bakelite, rubber.
Unit XVI: Chemistry in everyday life : (Period 8 )
1. Chemicals in medicines - annalgesica, tranquilizers, antisecptics, disinfectants, antimicrobials, antifertility drugs, antibiotics, antacids, antihistamines.
2. Chemicals in food- preservatives, artificial sweetening agents.
3. Cleansing agents - soaps and detergents, cleansing action.
CLASS XII (PRACTICALS)
STRUCTURE OF QUESTIONS PAPER
One Practical Paper Time : 3 Hours 30 marks
Experiments
Volumetric Analysis 10 Marks
Mixture Analysis 8 Marks
Content Based Experiment 4 Marks
Project 4 Marks
Class record & Viva 4 Marks
NOTE :-
Brief write up carrying 2 marks (If in the Question paper) question on preparation of crystals (Time for write up 5 minutes).
Stepevise distribution of marks of questions on salt analysis.
(i) Physical nature 1/2
(ii) Dry heating test 1/2
(iii) Flame test 1/2
(iv) Charcoal cavity test 1/2
(v) dil H2SO4 1
(vi) conc. H2SO4 test 1/2
(If anion is detected under dil H2SO4 test full credit of 1½ marks is to be given there and then)
Confirmalory test (any two ) 1½ marks each 3.
(vii) Preparation of original solution 1/2
(viii) Correct group detection 1
(ix) Systematic detection of ion 1
(x) Any two confirmatory tests of cation 2
( 1 marks each)
Step wise distribution of marks of questions on volumetric analysis.
Details of written part
Correct indicator ½
Correct end point ½
Balanced equation 2
Solution in burette 1
General calculations 1
5
Practical Part
Initial reading 1
Final reading 1
Correct use of pipetle ½
Correct end point ½
Three concordent readings ½
Correct titre value 1½
5
Full credit of + 2 % variation and deduct ½ mark for additional 0.1 ml variation.
PRACTICALS
Evaluation Scheme for Examination Marks
Volumetric Anaysis 10
Salt Analysis 8
Content Based Experiment 4
Class record and viva 4
Investigatory Project 4
Total: 30
Practicals Syllabus (Periods 6)
A. Surface Chemistry
(a) Preparation of one lyophilic and one lyophobic sol.
Lyophelic sol - starch, egg albumin and gum.
Lyophobic sol - aluminium hydroxide, ferric hydroxide, arsenious sulphide.
(b) Study of the role of emulsifying in stabilizing the emulsions of different oils.
B. Chemical Kinetics
(a) Effect of concentration and temperature on the rate of reaction between sodium thiosulphate and hydrochloric acid.
(b) Study of reaction rates of any one of the following:
(i) Reaction of iodide ion with hydrogen peroxide at room temperature using different concentration of iodide ions.
(ii) Reaction between potassium iodate, KlO3 and sodium sulphite : (Na2SO3) using starch solution as indicator (clock reaction).
C. Thermochemistry (Periods 6)
Any one of the following experiments
i) Enthalphy of dissolution of copper sulphate or potassium nitrate.
ii) Enthalphy of neutralization of strong acid (HCl) and strong base (NaOH)
iii) Determination of enthalpy change during interaction (Hydrogen bond formation) between acetone and chloroform.
D. Electrochemistry (Periods 2)
Variation of cell potential in Zn/Zn2+llCU2+/Cu with change in concentration of electrolytes (CuSO4 or ZnSO4) at room temperature.
E. Chromatography (Periods 2)
i) Separation of pigments from extracts of leaves and flowers by paper chromatography and determination of Rf values.
ii) Separation of constituents present in an inorganic mixture containing two cations only (constituents having wide difference in Rf values to be provided).
F. Preparation of Inorganic Compounds (Periods 4)
i) Preparation of double salt of ferrous ammonium sulphate or potash almum.
ii) Preparation of potassium ferric oxalate.
G. Preparation of Organic Compounds (Periods 4)
Preparation of any two of the following compounds
i) Acetanilide
ii) Di-benzal accetone
iii) p-Nitroacetanilide.
iv) Aniline yellow or 2-Napthol aniline dye.
v) Iodoform.
H. Test for the functional groups present in organic compounds: (Periods 6)
Unsaturation, alcoholic, phenolic, aldehydic, ketonic, carboxylic and amino (primary) groups.
I. Study of carbohydrates, fats and proteins in pure form and detection of their presence in given food stuffs. (Periods 4)
J. Determination of concentration/molarity of KMnO4 solution by titrating it against a standard solution of : (Periods 8 )
(i) Oxalic acid.
(ii) Ferrous ammonium sulphate.
(Students will be required to prepare standard solutions by weighing themselves).
K. Qualitative analysis (Periods 14)
l Determination of one cation and one anion in a given salt.
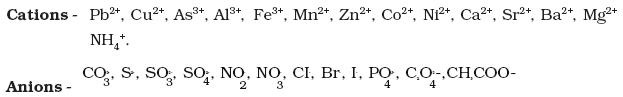
(Note: Insoluble salts excluded)
Few Suggested Projects
l Study of diffusion of a solid into a liquid.
PHYSICS SYLLABUS (10+2)
Theory Paper Time : 3 Hours Max. Marks: 70
Practical Paper Time : 3 Hours Max. Marks 30
Total Marks : 100
THEORY
STRUCTURE OF QUESTION PAPER
1. There will be one Theory Paper comprising of 30 questions. All questions will be compulsory.
2. Q Nos. 1-10 will be of 1 mark, Q.Nos. 11-18 will be of 2 marks each Q. No. 19-26 will be of 3-marks each, Q-Nos. 27 to 30 will be of 5 marks each.
3. In questions No. 27-30 there will be internal choice in all the four questions
4. Distribution of marks over different of the paper will be as follows:
Learning Outcomes Marks Percentage of Marks
i) Knowledge 20 29%
ii) Understanding 30 42%
iii) Application 20 29%
5. There will be no question of the type ‘Write short note on’ or objective type such as yes/no, tick, (x) cross’, fill in the blanks, multiple choice, true/false etc.
6. Weightage to units in the question paper can vary by one mark.
7. Use of unprogrammable calculator is allowed. The log tables can also be used.
8. Numerical problems can be et many type of questions but the total weightage willbe in the range of 25% to 30%.
CLASS XII (THEORY)
---------------------------------------------------------------------------------------------------------------------------------------------------------
One Paper Time : 3 Hours 70 marks
---------------------------------------------------------------------------------------------------------------------------------------------------------
Unit No. Title Marks
--------------------------------------------------------------------------------------------------------------------------------------------------------
Unit I Electrostatics 08
Unit II Current Electricity 07
Unit III Magnetic effect of currem & Magnetism 08
Unit IV Electromagnetic induction and Alternating current 08
Unit V Electromagnetic Waves 03
Unit VI Optics 14
Unit VII Deal Nature of Matter 04
Unit VIII Atoms and Nuclei 06
Unit IX Electronic Devices 07
Unit X Communication Systems 05
Total : 70
--------------------------------------------------------------------------------------------------------------------------------------------------------
Unit I: Electrostatics
Electric Charges; Conservation of charge, Coulomb’s law-force between two point charges, forces between multiple charges; superposition principle and continuous charge distribution.
Electrical field, electric field due to a point charge, electric field lines; electric dipole, electric field due to a dipole; torque on a dipolein uniform electric field.
Electric flux, statement of Gausss’s theorem and its applications to find field due to infinitely long straight wire, uniformly charged infinite plane sheet and uniformly charged thin spherical shell (Field inside and outside).
Electric potential, potential difference, electric potential due to a point charge, a dipole and system of charges; equipotential surfaces, electrical potential energy of a system of two point charges and of electric dipole in an electrostatic field.
Conductors and insulators, free charges and bound charges inside a conductor. Dielectrics and electric polarisation, capacitors and capacitance, combination of capacitors in series and in paralle, capacitance of a parallel plate capacitor with and without dielectric medium between the plates, energy stored in a capacitor Van de Graaff generataor.
Unit II: Current Electricity
Electric current, flow of electric charges in a metillic conductor, drift velocity, mobility and their relation with electric current; Onm’s law, electrical resistance. V-I characteristics (linear and non lineart), electrical energy and power, electrical resistivity and conductivity.
Carbon resistors, colour code for carbon resistors; series and parallel combinations of reastors; temperature dependence of resistance. Internal resistance of a cell, potential difference and emf of a cell,combination of cells in series and in parallel.
Kirchhoff’s laws and simple applications. Wheatstone bridge, metre bridge. Poentiometer - principle and its applications to measure potential difference and for comparing emf of two cells; measurement of internal reistance of a cell.
Unit III : Magnetic Effects of Current and Magnetism
Concept of magnetic field. Oersted’s experiment; Biot-Savart law and its application to current carrying circular loop.
Ampere’s law and its applications to infinitely long straight wire, straight and toroidal solenoids.
Force on a moving charge in uniform magnetic and electric fields. Cyclotron. Force on a current-carrying conductor in a uniform magnetic field. Force between two parallel current-carrying conductors, definition of ampere. Torque experienced by a current loop in uniform magnetic field; moving coil gal galvanometaits current sensitivity and
conversion to ammeter and voltmeter.
Current loop as a magnetic dipole and its magnetic dipole moment. Magnetic depole moment of a revolving electron. Magnetic field intensity due to a magnetic dipole (Bar magnet) along its axis and perpendicular to its axis. Torque on a magnetic dipole (bar magnet) in a uniform magnetic field; bar magnet as an equivalent solenoid, magnetic field lines; Earth’s magnetic field and magnetic elements. Para-, dia-and ferro-magnetic substances, with examples. Electromagnets and factors affecting their strengths. Permanent magnets.
Unit IV : Electromagnetic induction and Alternating Currents
Electromagnetic induction; Faraday’s law, induced emf and current; Lenz’s Law, Eddy currents. Self and mutual inductance.
Need for displacement current. Alternating currents, peak and rms value of alternating current/voltage; reactance
and impedances; LC oscillations (qualitative treatment only), LCR series circuit, resonance; power in AC circuits, wattless current. AC generator and transformer.
Unit V : Electromagnetics waves
Electromagnetic waves and their characteristics (qualitative ideas only). Transverse nature of electromagnetic waves.
Electromagnetic spectrum (radio waves, microwaves, infrared, visible, ultraviolet, Xrays, gamma rays) including elementary facts about their uses.
Unit VI: Optics
Reflection of light, spherical mirrors, mirror formula. Refraction of light, total internal reflection and its applications, optical fibres, refraction at spherical surfaces, lenses,thin lens formula, lens-maker’s formula. Magnification, power of a lens, combination of thin lenses in contact. Refraction and dispersion of light through a prism.
Scattering of light-blue colour of the sky and reddish appearance of the sun at sunrise and sunset.
Optical instruments: Human eye, image formation and accommodation, correction of eye defects (myopia, hypermetropia, presbyopia and astigmatism) using lenses. Microscopes and astronomical telescopes (reflecting and refracting) and their magnifying powers.
Waves optics: wave front and Huygens’ Principle, reflection and refraction of plane wave at a plane surface using wave fronts. Proof of laws of reflectionand refraction using Huygens’ principle. Interference, Young’s double slit experiment and expression for fringe width, coherent sources and sustained interference of light. Diffraction due to a single slit,
width of central maximum. Desolving power of microscopes and astronomical telescopes. Polarisation, plane polarised light; Brewster’s law, uses of plane polarised light and pointed.
Unit VII: Dual nature of Matter and Radiation
Dual nature ratiation Photoelectric effect, Hertz and Lenard’s observations’; Einstein’s photoelectic equation-particle nature of light.
Matter waves-wave nature of particles, de Broglic relation. Davission-Germer experiment.
Unit VIII; Atoms & Nuclei
Alpha-particle seating experiment; Rutherford’s model of atom;Bohr model, energy levels. hydrogen spectrum.
Composition and size of nucleus, atomic masses, isotopes, isobars; isotones.
Radioactivity-alpha, beta and gamma particles/rays and their properties; radioactive decay law. Mass-energy relation, mass defect; binding energy per nucleon and its variation with mass number; nuclear fission and fusion.
Unit IX: Electronic Devices
Semiconductors; semiconductor diode-I-V characteristics in forward and reverse bias, diode as a rectifier, I-V characteristics of LED, photodiode, solar cell and Zener diode, Zener diode as a voltage regulator, Junction transistor, transistor action, characteristics of a transistor: transistor as an amplifier (common emitter configuration) and oscillator, Logic gates (OR, AND, NOT, NAND and NOR). Transistor as a switch.
Unit X : Communication Systems
Elements of a communication system (block diagram only); bandwidth of signals (speech, TV and digital data); bandwidth of transmission medium. Propagation of electromagnetic waves in the atmosphere, sky and space wave propagation. Need for modulation. Production and detection of an amplitude-modulated wave.
CLASS XII (PRACTICALS)
One Practical Paper Time : 3 Hours 30 marks
Notes : All experiments are compulsory. The question paper will contain 8 experiments in all, 4 from each section. The examinee will have to mark three experiments from each section and the examiner will allot one experiment from each section.
2. Records of experiments are to be maintained.
3. Records of activities are to be maintained.
Two experiments 14 marks (7marks each)
Record of activities 3 marks
Viva on activities 3 marks
Record of experiments 5 marks
Viva experimens 5 marks
PRACTICALS
Note: Every student will perform 10 experiments 15 from each section and 6 activities (3 from each section) during the academic year.
SECTION A
Experiments :
1. To determine resistance per cm of a given wire by plotting a graph of potential difference versus current.
2. To find resistance of a given wire using metre bridge and hence determine the specifie resistance of its material.
3. To verify the laws of combination (series/parallel) of resistances using a metre bridge.
4. To compare the emf of two given primary cells using potentiometer.
5. To determine the internal resistance of given primary cell using potentiometer.
6. To determine reistance of a galvanometer by half-deflection method and to find its figure of merit.
7. To convert the given galvanometer (of known resistance and figure of meno into an ammeter and voltmeter of desired range and to verify the same.
8. To find the frequency of the a.c. mains with a sonometer.
Activities
1. To measure the resistance and impedance of an inductor with or without iron core.
2. To measure resistance, voltage (AC/DC), current (AC) and check continuity of a given circuit using multimeter.
3. To assemble a household circuit comprising three bulbs, three (on/off) switches, as fuse and a power source.
4. To assemble the components of a given electrical circuit.
5. To study the variation in potential drop with length of a wire for a steady current.
6. To draw the diagram of a given open circuit comprising at least a battery, resistor theostar, key ammeter and voltmeter. Mark the components that are not connected in proper order and correct the circuit and also the circuit diagram.
SECTION B
Experiments
1. To find the value of v for different values of u in case of a concave mirror and ........the local length.
2. To find the focal length of a convex lens by plotting graphs between u and v or between I/a and I/v.
3. To find the focal length of a convex mirror, using a convex lends.
4. To find the focal length of a concave lens, using a convex lens.
5. To determine angle of minimum deviationfor a given prism by plotting a graph between angle of incidence and angle of deviation.
6. To draw the I-V characteristic curve of a p-n junctionin forward bias and reverse bias.
7. To draw the characteristic curve of a zener diode and to determine its reverse breakdown voltage.
8. To study the characteristics of a common-emitter npn or pnp transistor and to find out the values of current and voltage gains.
Activities
1. To study effect of intensity of light (by varying distance of the source) on and L.D.R.
2. To identify a diode, an LED, a transistor, and IC, a resistor and a capacitor from mixed collection of such items.
3. Use of multimeter to (i) identify base of transistor. (ii) distinguish between npn and pnp type transistors. (iii) see the unidirectional flow of current in case of a diode and an LED. (iv) Check whether a given electronic component (e.g. diode, transistor or IC) is in working order.
4. To observe refraction and laterial deviation of a beam of light incident obliquely on a glass slab.
5. To observe polarization of light using two Polaroids.
6. To observe diffraction of light due to a thin slit.
7. To study the nature and size of the image formed by (i) convex lens (ii) concave mirror, on a screen by using a candle and a screen (for different distances of the candle from the lens/mirror).
8. To obtain a lens combination with the specified focal length by using two lenses from the given set of lenses.
Download Hindi Syllabus Class XII
- Science X 2008 - 3
(163 downloads)