Download CBSE Chemistry Sample Papers Class XI (11th) 2006
Sample Paper 2006
Chemistry
CLASS :XI
(set-1)
General instructions
- All questions are compulsory
- Do all the questions.
- All questions are of 5 marks.
- You have to attempt 5 marks from each question ________________________________________________________________________
Q1. (A) Name the metals which are associated with the following process:
1) Aluminothermy
2) Bessemer converter 1+1
Or
(A) Complete the following reactions:
RX + AgNO2 à ? + ?
RCOOAg + Br2 à ? 1+1
(B) Outline the principles of refining of metals by the following methods:
1) Electrolytic Refining
2) Zone Refining
3) Vapour Phase Refining
1+1+1
Or
(B) Give the general formula for
- Oxides of first group
- Halides of second Group
- Hydrides of first group. 1+1+1
Q2. (A) Differentiate between
- Precision and Accuracy
- Gram Molecular Mass and Gram Atomic Mass 1+1
Or
(A) How many moles of NaOH are in there 27 mL of 0.15 M NaOH? 2
(B) Zinc and Hydrochloric acid react according to the reaction:
Zn(s) + 2 HCl à ZnCl2 (aq) + H2(g)
If 0.30 mol Zn is added to hydrochloric acid containing 0.52 mol HCl, how many moles of H2 are produced? 3
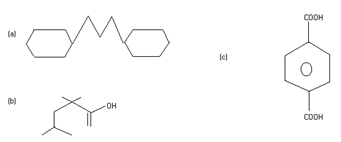
Q3. (A) Give the stick, condensed and bond line formulae for C4H10O. 2
Or
(A) Discuss the stability of Carbocations. 2
Or
(A) Explain
o Hyperconjugation Effect
o Electromeric Effect 1+1
(B) Give the IUPAC names of the following compounds:
Or
(B) Discuss the Permutit method for removal of the hardness of water. 3
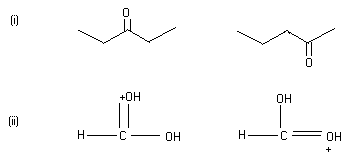
Q4. (A) What is the relationship between the members of the following pairs of structures? Are they identical, structural or geometrical, or resonance contributors?
Or
(A) Give the test for the quality detection for Nitrogen and Sulphur. 2
Or
(A) Explain that how the quantity of Halogen is detected in the Organic Compound. 2
(B) Explain one of the methods to determine the molecular mass of the organic compound. (It should not be Mass Spectrometry method). 3
Or
(B)An acid of molecular mass 104 contains 34.6% carbon and 3.85% Hydrogen. Suggest a structure for the acid. 3
Q5. (A) Calculate the density of ammonia at 300C and 5 bar pressure. 2
Or
(A) Differentiate between Ideal Gas and Real gas. 2
(B) Define
a) Vapour Pressure
b) Coefficient of Viscosity
c) Surface Tension 1+1+1
Or
(B) Define
a) Isobars
b) Isotones
c) Isotopes 1+1+1
Q6. (A) Explain the spectrum of the Hydrogen. 2
Or
(A) Comment:
a) Bohr’s Orbits are called Stationary Orbits
b) Energy of electron in nth orbit of Hydrogen is negative 1+1
Or
(A) Explain
a) Zeeman Effect
b) Stark Effect 1+1
(B) Differentiate between Absorption Spectrum and Emission Spectrum. 3
Or
(B) Calculate the energy of one mole of photons having frequency 5 * 1014 Hz. 3
Q7. (A) Which of the following pairs of elements would you expect to have lower ionization energy?
a) Cl or F
b) K or Ar 2
Or
(A) Give the Isoelectronic Species of Na+, Cl-, Fe3+ and Cu2+ 2
(B) Explain the variation of any two from Atomic Radii, Ionization Enthalpy and Electron affinity along a Period and a Group. 3
Download Attached PDF
- Scheme For General Hindi
(160 downloads)